
menu
You are about to leave the website of Otsuka Novel Products GmbH and view the content of an external website. Otsuka Novel Products GmbH cannot be held responsible for the content of external websites.
New tuberculosis (TB) compounds are discovered by Otsuka’s researchers in Tokushima, Japan, and are then advanced for clinical development.
In 2002, Otsuka’s researchers discovered a new compound that was effective against Mycobacterium tuberculosis, and it took 12 years of dedicated hard work to take the compound through clinical development and achieve the first marketing approval.
The last TB medicines had been developed about 50 years ago, and no medicine at the time of the compound’s trial had ever been approved specifically for multidrug-resistant TB (MDR-TB). Significant innovative capacity building was necessary in order to carry out a high-quality clinical trial.
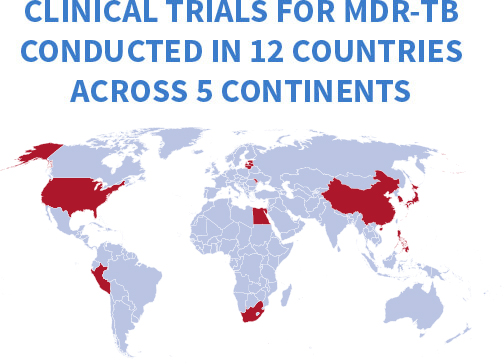
Increasing local capacity was key in areas such as data safety and monitoring, trial conduct, and laboratory services. Such activities strengthen the local health system and can benefit patients well beyond the duration of the trial.
You can learn more about Otsuka’s Research and Development activities for new TB medicines together with the key partners: PAN-TB Collaboration and Unite4TB.
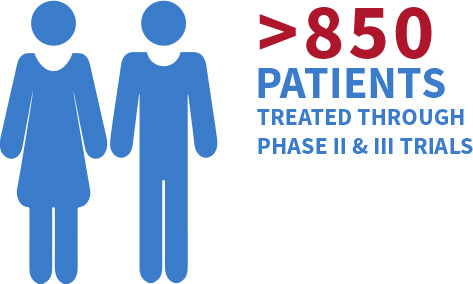
Since 2005, Otsuka has been one of the leading private sector funders in TB drug development.1
The company’s global TB franchise includes new drugs, treatment monitoring tools, paediatric innovations, diagnostics, and public health programmes that provide a comprehensive approach to fighting TB.

References
1. Based on Treatment Action Group, Reports on Tuberculosis Research Funding Trends, issues from 2006 until 2022 (accessed 01 June 2023). All reports available at: https://www.treatmentactiongroup.org/resources/tbrd-report/
Information current as of 16-06-2023
Reference ONP-DEL-2300011