
menu
You are about to leave the website of Otsuka Novel Products GmbH and view the content of an external website. Otsuka Novel Products GmbH cannot be held responsible for the content of external websites.
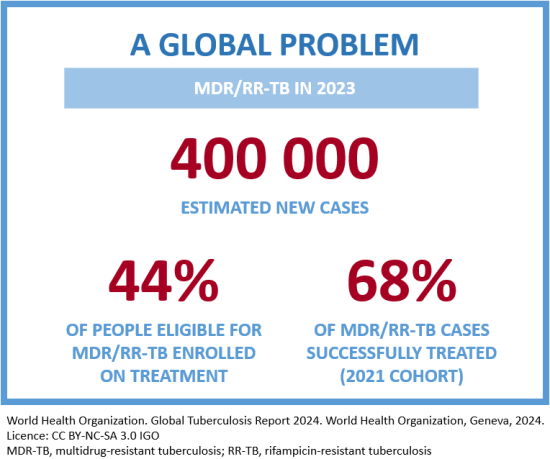
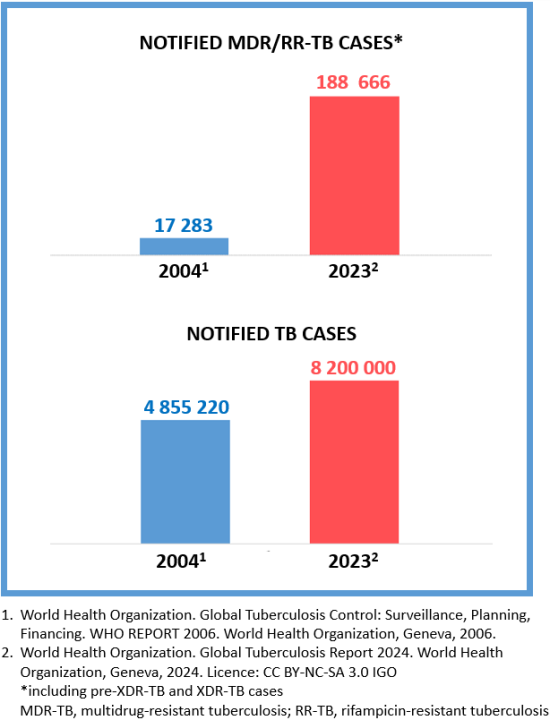
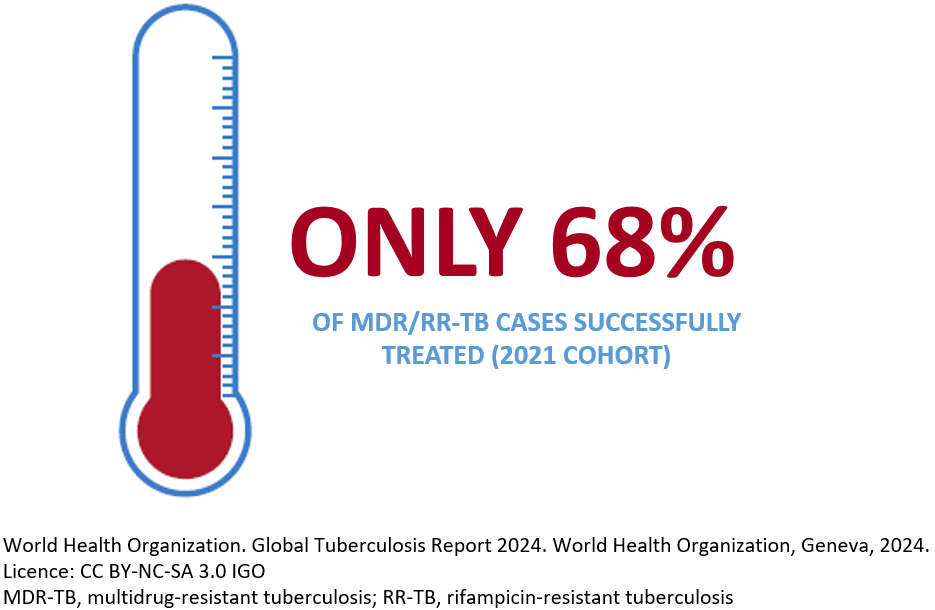
Bacteria can become resistant to any antibiotic due to inappropriate treatment, such as when it is interrupted or the medicine is used inappropriately.2 When the TB bacteria becomes resistant to at least isoniazid and rifampicin, it is called MDR-TB.1
Otsuka has had a TB medicine discovery programme for over 50 years and has been a recognised leader in TB research through its commitment to the development of new TB compounds as well as the building of a clinical infrastructure within developing countries affected by the disease.
Apart from continuing its research and development programme, Otsuka is also engaging in multiple third-party collaborations looking at shorter, more effective and more patient-friendly ways to fight MDR-TB.
One such collaboration will explore the safety and efficacy of Otsuka’s second anti-TB compound as part of an all-oral, Pan-TB regimen.
References
1. World Health Organization. Global Tuberculosis Report 2024. World Health Organization, Geneva, 2024. Licence: CC BY-NC-SA 3.0 IGO.
2. World Health Organization. Tuberculosis Factsheet. Available at: https://www.who.int/en/news-room/fact-sheets/detail/tuberculosis (Accessed 24 February 2025).
Information current as of March 2025
Reference ONP-DEL-2500005